|
| Note: References and ideas taken from NCERT book. special thanks to my middle school masterji🙏 all images are self prepared |
"It is a symbolic representation of a chemical equation."
Note:- The representation of a chemical reaction in words are collectively known as Word equation.
2 H2 + O2 -> 2 H2O (chemical equation)
In previous classes we have already deal with the variety of changes around us like melting of an ice cube, Formation of rust on a metal rod, conversion of milk into curd, burning of coal, Cutting of vegetable into small pieces,etc.
- All these changes may be physical or chemical.
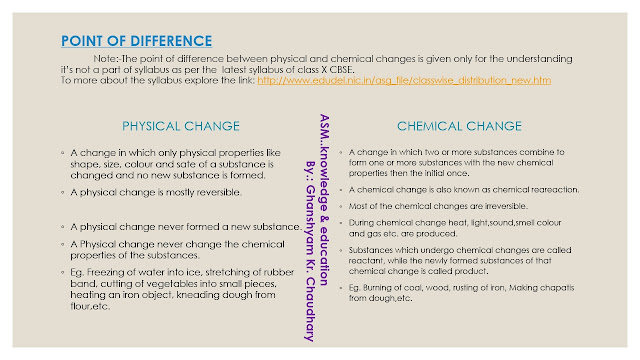
POINT OF DIFFERENCES
Que. i) Define the term chemical reaction and its
various type?
ii) Explain each type with suitable example?
Ans. A chemical reaction is a chemical changes in which one or more substances react to form a new substance which are entirely different from the initial substances which undergo chemical changes.
In a chemical reaction,
- The reacting species (i. e. molecule, atom, ions) are known as reactant.
- The new species or substance formed as a result of the chemical changes or a chemical reactions are known as product.
Eg. A + B -> C +D
Here: A, B are reactant
C, D are product
TYPES OF CHEMICAL REACTIONS
A chemical reaction may classified on the basis of chemical changes takes up and these are as follows:
COMBINATION REACTION
"A combination reaction is a type of chemical reaction in which two or more substances combine together to form a single product."
Eg. A + B -> AB
where: A, B = Reactant
AB = Product
Note:- Most of the combination reactions are exothermic.But also remember each and every combination reaction will not be an exothermic reaction.
DECOMPOSITION REACTION
"It is a type of chemical reaction in which a single substance breaks(i.e.decompose) to form two or more product."
Eg. AB -> A + B
Here, AB is a single reactant that breaks to form new substance(product)

- Decomposition reaction is also opposite to combination reaction.
Note:- All decomposition reactions are called endothermic reactions as it needs energy in different forms (as discussed below) for the formation of product either breaking the reactant.
- Types of Decomposition Reaction:
Eg. i) Thermal decomposition
ii) Electrolysis
iii) Photolysis or photochemical decomposition.
POINT OF DIFFERENCE
DISPLACEMENT REACTION
It may be 2 types
i) SINGLE DISPLACEMENT REACTION
"It is the type of chemical reaction in which a more reactive element reacts with a compound that bears a less reactive element and display it from its compound are called single displacement reaction."
Eg.
- It is because Fe has more reactivity than Cu thus it displace Cu from its salt.
ii) DOUBLE DISPLACEMENT REACTION
"It is a type of chemical reaction in which new substances are formed due to mutually exchange of ions between two compound."
- Double displacement reactions are also called precipitation reactions as precipitate(a solid substance) formed after such reactions.
Eg.
- Due to mutually exchange of ions among the compound a brisk white precipitate of BaSO4 is formed.
OXIDATION
It occurs when:-
i) An oxygen atom added to a substance.
ii) Hydrogen atom removed from a substance.
iii) A substance loses electron.
Note:- Oxidation always occurs from the left side of a chemical reaction(i.e. frome product side)
REDUCTION
It occurs when:-
i) An oxygen atom removed from a substance.
ii) Hydrogen atom added to a substance.
iii) A substance gains electron.
Note:- Reduction always occurs form the right side of a chemical reaction(i.e. from Reactant Side)
Now from the above points we can defined the OXIDATION-REDUCTION REACTION OR REDOX REACTION.
"Redox reaction or oxidation reduction reaction is a type of chemical reaction in which oxidation(i.e. addition of [O], and removal of [H ] with loss of electrons) and reduction(i.e. removal of [O],addition of [H] with gain of electrons) both taking place simultaneously are collectively known as oxidation reduction or redox reaction.
Note:-During the redox reaction if an element loses electron(i.e. gain of oxygen) is said to be oxidised while those have loses of oxygen is said to be reduced.
Eg.
HOMEWORK
Hello Dear learners are you know about the effect of oxidation in our daily life if yes then comment us about them, and if no then take your Class 10 NCERT BOOK chapter 1 on page 13 and read about it.
Qus. What are exothermic and endothermic reaction? defined each separately.
Ans.
Exothermic reaction: Chemical reaction in which heat is released either evolves with the formation of product are known as exothermic reaction.
Exothermic reaction are also called as combustion reaction.
Eg. CH4 + 2 O2 -> CO2 + 2 H2O + Heat
Note:- Respiration(cellular respiration) is also an exothermic reaction as it involves conversion of stored energy(glucose) to useful energy in the form ATP.
- C6H12O6 + 6O2 -> 6 CO2 + 6 H2O + Energy
Endothermic reaction: Chemical reaction in which heat energy is utilised or absorbed for the formation of product either breaking of reactants.
Δ
Eg. 2 Pb(NO3)2 --------> 2 PbO + 4 NO2 + O2
- Photosynthesis is an endothermic process (We will deals it with in Chapter 06 : Life Process.
#very_soon we will deals with part II chemical equations under which we will learn about the steps involves in balancing a chrmical equation, way of representing a chemical equations ,etc....
Thanks for reading🙏🇮🇳🙏
If it is useful then Don't forget to like and if it is worst than you also have a choice to dislike.
Don't forget to comment beacuse it is a valuable suggestion from your side to us for improvement.
Radhey Radhey
Follows us on Facebook Page,
"ASM a place of free education & knowledge."